Halogenated flame retardants have been widely used in the flame retardant modification of polypropylene for their high-efficiency flame retardant effect, but their toxic and highly corrosive gas hydrogen halide released during the flame retardant process will be environmentally friendly. Human health causes great harm. It is of great significance to develop clean, efficient, safe, environmentally friendly, low-cost flame retardants and fire-safe flame retardant polymer materials. Halogen-free flame retardant technology is a development trend in the field of flame retardant in recent years, and has attracted the attention of the industry.
Industry standard for halogen-free flame retardant products

Xiaobian today will introduce the principle and main methods of the current halogen-free flame retardant modified polypropylene.
Phosphorus flame retardant
The phosphorus-based flame retardant system includes an inorganic phosphorus-based flame retardant and an organic phosphorus-based flame retardant. The most widely used organophosphorus flame retardants are phosphates and phosphonates such as triphenyl phosphate, tris(xylylene) phosphate, triethyl phosphate, triisopropylphenyl phosphate, trioctyl phosphate, Tolyl diphenylphosphonate, tris(β-chloroethyl) phosphate, tris(2,3-dibromopropyl) phosphate, tris(dibromophenyl)phosphate, etc. Red phosphorus (microencapsulated red phosphorus), ammonium polyphosphate, phosphate (such as diammonium hydrogen phosphate, ammonium dihydrogen phosphate, ammonium phosphate, etc.).
Flame-retardant mechanism: Phosphorus-based flame retardant is a weak flame retardant; its flame-retardant mechanism is that the phosphorus-based compound is thermally decomposed into phosphorus-containing oxyacids, which can promote the coke formation of the polymer by dehydration and charring. It can block the contact of the internal polymer with oxygen; the thermal conductivity of the coke layer is poor, which insulates the polymer from the heat source, slows down the thermal decomposition, and thus acts as a flame retardant. The step of dehydration carbonization must rely on the oxygen-containing groups of the polymer itself. In the case of polypropylene, the molecular structure has no oxygen-containing groups, and the flame retardant effect is poor when the phosphorus-based flame retardant is used alone. If it is compounded with aluminum hydroxide and magnesium hydroxide, a synergistic effect can be produced, thereby obtaining a good flame retardant effect.
Commonly used formula: Phosphorus-based flame retardants commonly used in polypropylene are mainly red phosphorus and organophosphorus compounds (such as phosphate esters). Studies have shown that the flame retardant effect of Mg(OH)2 coated red phosphorus compound is better than that of flame retardant polypropylene with Mg(OH)2 alone, but the mechanical properties are degraded. The compounding of 10 parts of red phosphorus and 80 parts of Mg(OH)2 has a clear synergistic flame retardant effect, so that the oxygen index of the system reaches 29%, and the comprehensive performance is good.

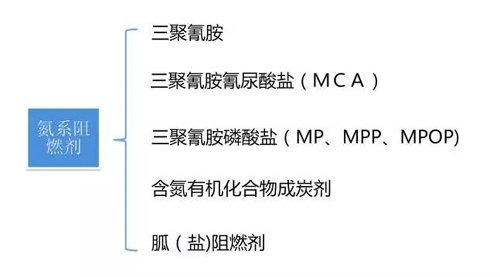
2. Nitrogen flame retardants
Nitrogen-containing flame retardants mainly include three major categories: triazines melamine, dicyandiamide, cerium salts (cerium carbonate, cerium phosphate, cerium condensate cerium and sulfamate sulfa) and their derivatives, especially phosphates. derivative.
Flame-retardant mechanism: When the temperature rises to a certain extent, the nitrogen-based flame retardant is heated and begins to decompose. The decomposition products are NO, NO2, NH3, H2NCN, N2, H2O, CO2 and other non-combustible gases, of which NH3 is released. The main component of the gas, NH3 has the function of cooling, endothermic and diluting oxygen. The formation of these incombustible gases serves the purpose of absorbing heat and oxygen, and the endothermic reaction of the flame retardant in the decomposition process consumes most of the heat, which significantly reduces the temperature of the burning surface of the flame retardant material, and prevents the material from further The role of burning and spreading flames. The non-combustible gas substance not only plays the role of diluting the concentration of oxygen and flammable gas at the combustion interface, but also consumes oxygen in the air, reacts with it to generate nitrogen, water and deep oxides to achieve flame retardant. purpose.
Commonly used formula: Melamine (MA) is a representative of nitrogen-based flame retardants. It is easy to sublimate when heated. It is non-flammable and has a decomposition temperature of 250-450 ° C. The decomposition reaction is an endothermic reaction, which absorbs a large amount of heat and releases N2, NH3 and CN. -gas. MA helps the polymer to char and affects the melting behavior of the polymer. As a foaming component (air source) in the intumescent flame retardant, it has a good foaming effect and is densely formed into carbon, and is often used for flame retardant polypropylene. In addition to being used alone, MA is often reacted with an acid to form a nitrogen-derived salt-based flame retardant. Such derivative salts, such as M-series flame retardants (Ciba Refined Development), are thermoplastic in PP, PE, and PVC. In the field of thermosetting plastics, it is widely used; in addition, dicyandiamide which can replace melamine is mainly used for the manufacture of cerium salt flame retardant, or dicyandiamide is combined with melamine to synergistically flame retardant.
3. Silicon-based flame retardant
Silicon-based flame retardant is a new type of halogen-free flame retardant. It is a carbon-based smoke suppressant. It can improve the processing performance and improve the polymer while giving the polymer excellent flame retardancy and smoke suppression. The mechanical strength of the polymer. Silicon-based flame retardants are highly efficient, non-toxic, low-smoke, non-melting, and non-polluting. They are attracting attention in many halogen-free flame retardant systems. Silicon-based flame retardants are divided into two major categories: silicone and inorganic silicon. Silicone is mainly silicone, including silicone oil, silicone rubber, various siloxane copolymers and silicone resins. It is an organopolysiloxane; inorganic silicon is mainly composed of silicate (such as montmorillonite), silica gel, talc, and the like.
Flame-retardant mechanism: The silicone-based flame retardant will melt and drip earlier when it burns, and the droplets of the silicone flame retardant transfer to the surface of the substrate through the gap of the polymer matrix to form a dense The stable silicon-containing (main component SiO2) carbon layer, which contains the silicon-carbon layer, prevents the escape of the combustion-decomposed flammable substance, and also acts as a heat-insulating oxygen barrier to prevent the heat of the polymer material. Decomposition, to achieve the purpose of flame retardant, low smoke, low toxicity; the flame retardant effect of inorganic silicon flame retardant belongs to the condensed phase flame retardant mechanism, which is generally considered to be the barrier shielding of amorphous silicon or silicide protective layer formed by combustion. The role to achieve the purpose of flame retardant.
Commonly used formula: a reactive silicone-based flame retardant is connected as a polymerized monomer to a polymer molecular chain, or an organosiloxane is grafted onto a polymer molecular chain to realize a copolymer of a flame retardant and a matrix material. On the one hand, the flame retardant is prevented from migrating in the polymer, and on the other hand, the flame retardant is perfectly compatible with the polymer matrix, and the mechanical properties of the material can be effectively maintained or even improved. For example, SFR-100 polysiloxane polymer, similar to the binding mechanism of the polymer and the cross-linking mechanism of the interpenetrating polymer network, can significantly inhibit the fluidity of the silicon flame retardant in the polymer matrix. So that it does not migrate to the surface of the flame retardant material. Because SFR-100 has very good compatibility with polypropylene, it is often used alone or in combination with other synergists (aluminum hydroxide, ammonium polyphosphate, magnesium stearate, etc.) to flame retard polypropylene, which can improve PP. Smoke suppression and flame retardancy.
Inorganic silicon-based flame retardants which have been developed and widely used are SiO2, microporous glass, low-melting glass, and glass fiber, silicone gel/K2CO3, silicate/APP, SiO2/SnCl4, siloxane/boron, Hydrated silicon compound / APP, etc. The inorganic silicon-based flame retardant is represented by silica. SiO2 has both flame retardant effect and a polypropylene reinforcing additive; SiO2 usually cooperates with other flame retardants, for example, SiO2 and 1-oxyl. A silicon-phosphorus composite system formed by pyro-4-hydroxymethyl-2,6,7-trioxabicyclo[2.2.2]octane (PEPA) is a flame retardant PP, a double ring with symmetrical structure synthesized by SiO2 and pentaerythritol. Cage-like four-coordinated silicon flame retardant, applied to PP, all play a good synergistic flame retardant effect. Co-efficient flame retardant of SiO2 with an intumescent flame retardant and magnesium hydroxide has also been studied for use in polypropylene.
4. Aluminum-magnesium flame retardant
The aluminum-magnesium flame retardant used for polypropylene is mainly aluminum hydroxide or magnesium hydroxide. These materials have the advantages of good thermal stability, non-toxicity, non-volatilization, no corrosive gas, and low smoke generation. However, in the polymer material, the amount of addition is large, and when the filling amount is more than 50% (wt), the polymer material has a certain flame retarding effect. Due to poor compatibility with polypropylene, it is difficult to uniformly disperse in polypropylene, and high filling amount will definitely affect the mechanical properties of polypropylene. At present, surface modification treatment, synergistic composite technology and ultra-fine particle size are the main research directions of aluminum-magnesium flame retardants.
Flame-retardant mechanism: The thermal decomposition process of aluminum hydroxide is an endothermic reaction (heat absorption is about 2kJ/g), which can take away a lot of heat generated by combustion and reduce the temperature of the combustion interface; in addition, one of the decomposition products is water. Vapor, which can reduce the temperature and dilute the concentration of oxygen and flammable gas; another decomposition product, alumina, is a dense inorganic oxide powder that can be covered on the surface of the polypropylene flame retardant material to form a layer of insulating oxygen barrier. The role of the protective carbon layer, but also has the effect of suppressing smoke.
The flame retardant mechanism of magnesium hydroxide is similar to that of aluminum hydroxide, but the decomposition temperature of magnesium hydroxide is 340~490°C, which is much higher than that of aluminum hydroxide, and the thermal stability is relatively good. It is also superior to hydrogen in smoke suppression and smoke suppression performance. Alumina, and the heat absorption of the reaction is less than that of aluminum hydroxide. In addition, Mg(OH)2 can promote the carbonization of the plastic surface, while Al(OH)3 has no such effect. At the same addition amount, there is no significant difference between the two on the flame retardant effect of polypropylene. However, Mg(OH)2 is more suitable as a flame retardant for polymers with higher processing temperature than Al(OH)3; Generally, the synergistic effect of the two flame retardants can achieve better flame retardant effect than the single use. The synergistic flame retardant mechanism is that the aluminum hydroxide exhibits a low flame retardant temperature, but the heat absorption is large, and the temperature can be effectively suppressed. Rising, when the temperature is high, the dehydration and endothermic reaction of magnesium hydroxide acts as a flame retardant, and the two combine to enhance the length and avoid the shortness, widen the temperature range of the flame retardant effect and prolong the time of exerting the flame retardant effect, thereby Effect of flame retardant.
Commonly used formulas: Studies have shown that magnesium hydroxide with zinc borate, expanded graphite, red phosphorus, montmorillonite and other synergistic flame retardant polypropylene, the two can work together to achieve better flame retardant performance than their own individual; hydrogen The particle size and properties of alumina and magnesium hydroxide are closely related. The smaller the particle size, the larger the specific surface area, and the better the flame retardant effect. For example, in the technology of refining aluminum hydroxide, Solem Company of the United States is Halofree's halogen-free, low-smoke, non-toxic, non-corrosive and high thermal stability flame retardant, which is based on modified aluminum hydroxide. Inorganic flame retardant.
At present, magnesium hydroxide has been developed and applied with nanometer magnesium hydroxide, granular magnesium hydroxide and fibrous magnesium hydroxide. The diameter is 0.1~0.5um and the length is 10~50um, which has both reinforcing and flame retardant effects. And does not affect the processing performance. The ultrafine inorganic powder has a relatively high surface energy and is easily agglomerated, and has poor dispersibility in the polymer matrix material, so it is required to be surface-activated and modified. Surface activation mainly involves surface treatment of Al(OH)3 and Mg(OH)2 with silane or titanate coupling agents to improve the dispersion and compatibility of inorganic powders in polymer matrix materials. .

5. Intumescent flame retardant
Intumescent flame retardant (IFR) is considered to be one of the development directions of halogen-free flame retardant materials due to its high flame retardant efficiency, low smoke, non-toxic, non-corrosive gas release. However, due to the poor compatibility of the intumescent flame retardant with polypropylene, moisture absorption, easy precipitation, etc., the surface modification of intumescent flame retardants, the synergistic effect of different flame retardants, and the development of new intumescent flame retardants have become The main development trend of intumescent flame retardant technology.
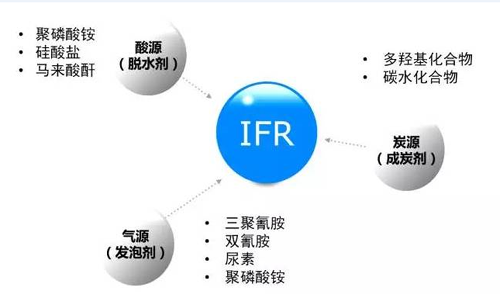
Flame Retardant Mechanism: IFR is generally a new composite flame retardant with phosphorus, nitrogen and carbon as main components. It usually consists of three parts - a charcoal (carbon source), a dehydrating agent (acid source) and a swelling agent (air source). In the conventional IFR composite flame retardant, ammonium phosphate (APP) is usually used as the acid source, pentaerythritol (PER) is used as the carbon source, and melamine (MA) is used as the gas source. When the temperature rises, the carbon source in the IFR undergoes an esterification reaction under the action of an acid source, and the product is an ester compound; thereafter, a dehydration crosslinking reaction occurs, the ester compound forms a carbonization, and the gas source decomposes. The generated gas acts on these chars to form a closed, porous, fluffy foamed structural carbon layer, which is essentially carbon crystallites, is an amorphous carbon structure, cannot be burned, and can function as a barrier polypropylene. The heat conduction between the burning material and the heat source causes the thermal degradation temperature of the poly flame retardant propylene to be improved. In addition, the closed foamed carbon layer can hinder the diffusion of gas, that is, prevent the flammable gas generated by thermal decomposition from diffusing, and block the external oxygen from flowing to the surface of the uncracked flame-retardant polypropylene material, due to insufficient combustion. Oxygen and heat, the burning flame retardant polypropylene material will self-extinguish.
Commonly used formula: By adjusting the ratio of the three components of carbon source, acid source and gas source, a composite type IFR with different flame retardant effects can be synthesized. The traditional composite IFR formula mixes the acid source, carbon source and gas source in a mass ratio of 3/1/1, and the resulting IFR has the best flame retardant effect. At present, polyhydroxy compounds and carbon-rich polymers are mainly used as carbon sources. The former mainly includes pentaerythritol, mannitol or sorbitol, and the latter includes thermoplastic polyurethane and PA6 as well as starch. Triazine polymers and their derivatives, which contain a stable triazine ring, have a carbon-producing property, and have become a novel carbon-forming agent, and have recently attracted attention in recent years. Ammonium polyphosphate, zinc borate and magnesium ammonium phosphate are the most common sources of acid. Melamine, dicyandiamide formaldehyde resin, boric acid amine, dicyandiamide, ammonium polyphosphate, chlorinated paraffin, etc. are commonly used gas source materials. The research on compound IFR is relatively mature. The main commercializations are CN-329 of Great Lake, MF-80 of Mented, Spinflam series of CN-197 and Monsato, Exolit of Chemest and Chemie Linz of Hochest. Melapur PA-90.
The one-component IFR carbon source, acid source and gas source are in the same component, which has the advantages of not only effectively reducing the amount of IFR added in the PP, but also improving the water absorption of the material, and having good thermal stability. In addition, graft copolymerization with a monomer of a polypropylene matrix material can be used to effectively improve the compatibility between the IFR and the polypropylene matrix, but the flame retardancy efficiency of the one-component IFR is relatively low. At present, the single-component IFRs at home and abroad are commercialized with fewer varieties. The first component IFR that was first applied to polyolefin flame retardant is 1-oxyphosphazene-4-hydroxymethyl-2,6,7- Phosphate ester of trioxabicyclo[2.2.2]octane (PEPA). Since then, the application and development of such compounds as an IFR flame retardant in polyolefins has attracted great attention in the flame retardant world. Pentaerythritol diphosphate melamine salt (PDM) is a representative of the pentaerythritol series of one-component IFR. It is the most typical "trinity" IFR flame retardant. It is also a commercial one-component IFR product with good flame retardant properties. Has good thermal stability and light aging resistance.
In order to further improve the flame retardant efficiency of IFR, zeolite, conventional metal compounds, metal chelates, natural clays and rare earth oxides are generally used as synergistic flame retardant aids for the IFR system. In addition, nanoparticles such as montmorillonite, hydrotalcite, single-walled carbon nanotubes or multi-walled carbon nanotubes are also added to the polypropylene matrix to work with IFR to enhance its flame retardant effect. In short, the addition of appropriate amount of synergist, combined with IFR flame retardant, can significantly improve the flame retardant properties of polypropylene, inhibit the release of heat during the combustion of polypropylene, slow the rate of heat release, while reducing the occurrence of burning The mass loss caused by the thermogravimetric effect increases the thermal stability of the polypropylene and increases the amount of carbon remaining after combustion.
In view of the problems of poor thermal stability, easy moisture absorption, poor compatibility with polypropylene, and insufficient flame retardant efficiency of IFR, new processing technologies such as nanocrystallization, surface modification, and microencapsulation have been used to improve the performance of IFR in recent years.
to sum up
Although the above-mentioned halogen-free flame retardant can achieve the effect of environmentally-friendly and high-efficiency flame retardant, there are still some problems. For example, commonly used aluminum-magnesium flame retardants are powdery, and exhibit excellent flame retardancy when added in a relatively large amount. Due to poor compatibility with polypropylene, the mechanical properties of flame retardant polypropylene are often made. The performance is difficult to meet the requirements of use; the intumescent flame retardant is easy to absorb moisture, and the poor compatibility with polypropylene easily leads to precipitation from the product, affecting the flame retardant performance and other performance; some nano materials are combined with polypropylene, and the mechanical properties are improved. However, the improvement in flame retardant performance is limited.
Therefore, research on the synergistic effect of new halogen-free flame retardants and different flame retardants, and develop new surface modifiers and new surface modification technologies to make flame retardants and polypropylene have suitable compatibility and construct moderately. The flexible and strong interfacial structure is the development direction of the halogen-free flame retardant polypropylene with excellent flame retardant performance and obviously improved mechanical properties, so that the halogen-free flame retardant polypropylene can adapt to the requirements of many fields as soon as possible, and enhance production. The safety of life.
Fireproof Coating,Tunnel Fireproof Coating,Fireproof Paint For Concrete,Fireproof Paint For Steel Beams
Jiangxi Long Zheng Techinical Developing (Pty) Ltd. , https://www.jxlongzhengkeji.com